Growing crystals is actually pretty easy to do at home and makes a great science and chemistry experiment for kids. This growing borax crystal hearts experiment for Valentine’s Day makes a great science activity and decoration to try with kids. We have tons of fun Valentine science experiments you are sure to love!

Valentine’s Day Science
This borax crystal heart science experiment is a set-it-up-and-forget-about-it kind of experiment like our crystal snowflakes. Growing crystals is a classic science experiment that you must try with your kids!
You can get creative and make any shape for your crystals to grow! Here are a few of our favorites…
Make sure to check out the video below and see it in action.
Crystal Hearts Experiment
NOTE: Adult assistance will be needed. Since you are dealing with hot water, my son watched the process while I measured the solution and stirred it. Borax is also a chemical powder best used by an adult for safety.
If you want a more hands-on type of crystal experiment, try our salt crystal hearts.
SUPPLIES:
- Borax
- Jars or vases (glass jars are preferred over plastic cups)
- Popsicle sticks
- String and tape
- Pipe cleaners
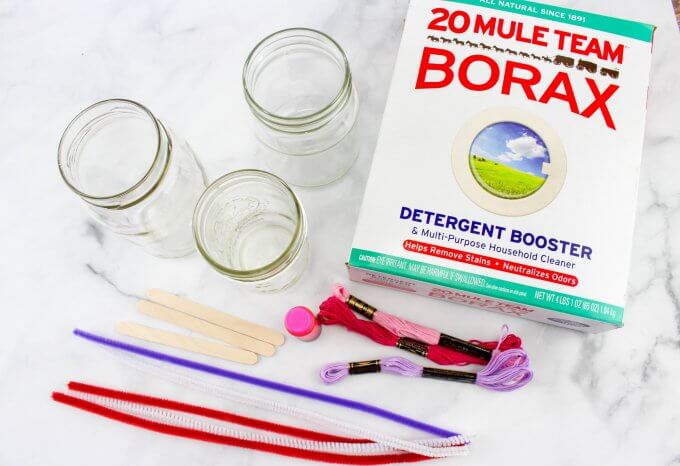
CRYSTAL HEARTS SET UP
STEP 1: Take your pipe cleaners and form them into hearts! Twist two different colors together! Or you can entwine two hearts!

Hint: Double-check the jar’s opening with the size of your shape! It’s easy to push the pipe cleaner in to start but challenging to pull it out once all the crystals have formed! Make sure you can get your heart in and out easily!
STEP 2: Use the popsicle stick (or pencil) to tie the string around. I used a small piece of tape to keep it in place. You can do two hearts in one jar, but make sure they are small and have room! They would also look pretty if they grew together!

STEP 3: MAKE YOUR BORAX SOLUTION
The ratio of borax powder to boiling water is 3:1. You want to dissolve three tablespoons of borax powder for each cup of boiling water. This will make a saturated solution, which is a great chemistry concept.
Since you need to use boiling hot water, adult supervision and assistance are highly recommended.

STEP 4: Make sure the heart is fully submerged in the solution!

Shhhh…
The crystals are growing!
You want to set the jars in a quiet place where they won’t be disturbed. No tugging on the string, stirring the solution, or moving the jar around! They need to sit still to work their magic.

After a couple of hours, you will see some changes. Later on that night, you will see more crystals growing! You want to leave the solution alone for 24 hours.
Make sure to keep checking to see the stage of growth the crystals are in!

The next day, gently lift out your crystal heart ornaments and let them dry on paper towels for an hour or so…

The Science of Growing Crystals
Crystal growing is a neat chemistry project that is quick to set up and involves liquids, solids, and soluble solutions. Because there are still solid particles within the liquid mixture, the particles will settle to form crystals if left untouched.
Water is made up of molecules. When you boil the water, the molecules move away from one another. Boiling hot water allows more borax powder to dissolve to create the desired saturated solution.
You are making a saturated solution with more powder than the liquid can hold. The hotter the liquid, the more saturated the solution can become. This is because the molecules in the water move farther apart, allowing more of the powder to be dissolved. If the water is colder, its molecules will be closer together.
Do you love chemistry… make sure to check out all our chemistry experiments!

Saturated Solutions
As the solution cools down, there will suddenly be more particles in the water as the molecules move back together. Some of these particles will start to fall out of the suspended state they were once in.
The particles will then settle on the pipe cleaners and the container and form crystals. Once a tiny seed crystal is started, more falling material bonds to form bigger crystals.
Crystals are solid with flat sides and symmetrical shapes and will always be that way (unless impurities get in the way). They are made up of molecules and have a perfectly arranged and repeating pattern. Some might be bigger or smaller, though.
Let your crystal hearts work their magic overnight. We were all impressed by what we saw when we woke up in the morning! We had quite the pretty crystal hearts Valentine’s science experiment!
Go ahead and hang them in the window like a suncatcher!

Growing Crystals in the Classroom
We made these crystal hearts in my son’s 2nd-grade classroom. This can be done! We used hot water but not boiling from a coffee urn with a spout and plastic, clear party cups. The hearts needed to be smaller or fatter to fit in the cup.
Plastic cups are generally not recommended for growing the best crystals, but the kids were still fascinated by crystal growth. When you use plastic cups, the saturated solution can cool too quickly, leaving impurities to form in the crystals. The crystals will not be as sturdy or perfectly shaped. If you can use glass jars, you will have better results.
Also, you need to make sure the kids really don’t touch the cups once they have gotten everything together! The crystals need to remain very still to form properly. Once set up, I recommend making sure you have space set up away from everything to fit the number of cups you have.
Free Printable Valentine STEM Calendar & Cards
More Valentine’s Day Science Experiments
For our complete collection of Valentine science experiments and activities, check here.






Printable Valentine STEM Project Pack
Countdown to Valentine’s Day with science and STEM! Pack includes complete instructions, templates, and images for 20+ activities. Bonus: printable science Valentine’s Day cards!










How much Borax {found with laundry detergent} to Water does one use to make the crystal from?
Please click through the recipe to Happy Hooligans to get the exact amounts. She is the original recipe holder!
Would it work to use plastic solo cups or only glass?
Borax is not available in the UK due to its toxicity do you know what could be used as a substitute?
Check out Fun At Home With Kids UK Alternative.
That would be an experiment!
I would try sugar – would take a bit longer, but I remember making Rock Candy as a kid, so I think it would work. Try this “recipe”: https://sciencebob.com/make-your-own-rock-candy/
You definitely could try sugar! I have had difficulty making rock candy here but will keep trying. This recipe works every time!
Very cute idea!
How much borax?
I apologize but since I found the recipe on another site, etiquette requires me to send you to her page to get the measurements. You should see a link asking you to visit Happy Hooligans. Let me know if you have trouble.
Did you try different cups? I used the disposable travel coffee cups & was an epic fail but 20 glass jars is not feasible in my classroom :-/
Ooh I have not tried different cups. What happened when you tried the coffee cups? It would be a great experiment to add to the list. I am sorry you can’t do all twenty. I have seen it done in one of those clear plastic storage containers. You can use dowels or paint sticks to lay across the top. That way you can hang several from one stick! Hope that works!
They only have about 4 crystals on them and I have dipped twice… boiled water in a tea kettle then added borax until disolved then transferred to cups. Storage tub is worth a try…. just disappointed that mine look NO where near as crystallized as yours. The cups were not clear.
That is disappointing but I have only ever seen them done with glass. Maybe glass mixing bowls if you have any might work or even ceramic baking dishes might be worth a try. I am trying to search around for an explanation, but all I can think of is that the material of the cup disrupted the suspensions process. I wonder if it might have been because the cup was insulated being a coffee cup that it didn’t settle right. Let me know what happens!
What about cleanup? What do you do with the solution after? And how do you get the crystals off the glass?
Hi! I fill the jars with hot water. It usually breaks up easily. I throw the big chunks away and wash away the rest. I run the jars through the dishwasher but reserve them for science experiments only.
Was thinking, perhaps the teacher looking for a way to do with the whole class could find someone with an aquarium they are not using to make them in.
Thanks!
Are they toxic or unsafe to be handled by school age children after they are made? Asking for 8+ age group.
No they can be handled by kids. I would wash hands thoroughly and of course you don’t want them handled too much as they are somewhat fragile.
To get 20 glass containers, save spaghetti sauce jars or ask for them on freecycle.org if your city/county has a site.
Make sure you get the water as hot as possible. Hot water will dissolve more Borax, and your crystals will grow bigger and better. Also, the slower it cools, the bigger your crystals will be. I did this project a few years ago, and showed a video about the formation of crystal caves first. We studied how crystals form, and made Christmas ornaments instead of Valentines gifts. It worked great. We then decorated the crystallized jars with ribbon, put tea lights inside, and viola, another gift for the parents! I did this with a big class, used some of my old caning jars, and then put the jars into big, deep trays from the kitchen so they wouldn’t spill. We also used some baking dishes to make crystallized coffee filter snowflakes, which were also sent home as ornaments,. We used the same solution, but the crystals there were smaller. It took up a lot of space in my classroom, but the results were worth it! I’m thinking that maybe the cups didn’t work because they cooled too quickly, or maybe there just wasn’t enough borax in the suspension?
Yes, you should use boiling water unless you are in the classroom and then we managed with a coffee urn of hot water.
The recipe calls for 1T borax to 1C water. I used mason jars, which are 2C, so ainused 2T borax. The experiment did not work. Martha Stewarr has a recipe that uses 2T per 1C, that I will tey.
I’m sorry we had a typo and it should have read 3:1 which would be three tablespoons like our other crystal recipes!