Atoms are tiny but very important building blocks of everything in our world. What are the parts of an atom? Learn the parts of an atom with an easy hands-on physics activity. All you need is some playdough or clay, and our printable parts of an atom worksheets to get started!

What Are The Parts Of An Atom?
Everything is made of matter, and all matter is made of atoms. Atoms are the building blocks of everything! They are so small that you can’t see them with your eyes, but they make up everything around us.
There are 3 parts of an atom, which are even smaller particles called protons, neutrons, and electrons.
Protons
Protons are found in the center of the atom, called the nucleus, and are the same size as neutrons.
As the atom is the smallest unit of matter that has the chemical properties of an element, the number of protons determines what element it is. For example, all hydrogen atoms have one proton, while all helium atoms have two.
Neutrons
The neutrons are also found in the center of the atom, or the nucleus. Protons and neutrons are the same size.
The number of neutrons in an atom can be different to the number of protons. The number is calculated by finding the Atomic Mass minus the Atomic Number. (You can find the number of proton, neutrons and electrons for some common elements at the end!)
Sometimes an element will have isotopes. Isotopes are atoms of the same element that contain the same number of protons but different numbers of neutrons.
Electrons
Electrons are much smaller than protons and neutrons, and orbit around the nucleus, kind of like the planets orbit around the sun. The random orbits of electrons are sometimes referred to as an electron cloud because the electrons are in constant motion, so the atom has no distinct outer edge.
The electrons in an atom are arranged in shells that surround the nucleus, with each shell being farther from the nucleus. The shell closest to the nucleus, can hold two electrons, while the next shell, can hold eight, and the third shell, can hold up to eighteen.
Protons have a positive charge, electrons have a negative charge, and neutrons have no charge. In order for the charge of an atom to be neutral, there must be the same number of protons as electrons.
Additionally, learn about the role of electrons in static electricity!
Using The Periodic Table
The periodic table is a chart that displays all of the known chemical elements. Each element is identified by its symbol, atomic number, and atomic mass. The periodic table allows scientists to predict the properties of elements and how they will react with other elements.
You can use the Periodic table to determine the atomic number of an element. The atomic number is the number of protons in the nucleus of an atom. Each element has a different number of protons, which gives it, its place in the periodic table.
The number of neutrons in an atom can be different to the number of protons. It is calculated by finding the Atomic Mass minus the Atomic Number.
Get your FREE Parts of an Atom Worksheets!
Build An Atom Project
Learn the parts of an atom using play dough to make electrons, neutrons, and protons and put them in their proper place.
Supplies:
- Build an atom worksheets
- Playdough or modelling clay in 3 different colors
NOTE: Check out our super easy no-cook playdough recipe to make your own!
Instructions:
STEP 1. Print out the build an atom worksheets and choose what colors you will use to represent the protons, neutrons and electrons.
We used blue – protons, pink – neutrons, orange – electrons.
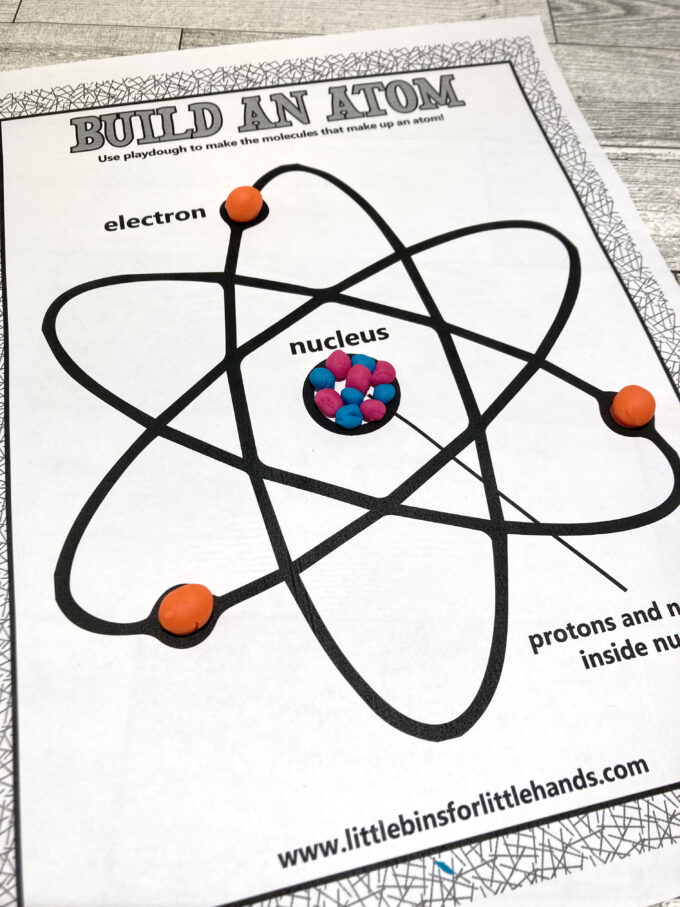
STEP 2. Add 5 protons to the center to make a Boron atom.

STEP 3. Add 5 or 6 neutrons to the nucleus. The most common isotope of Boron has 6 neutrons.

STEP 4. Add 5 electrons to the electron cloud around the center.
TIP: Remember to space the electrons out evenly, and make them much smaller than the protons and neutrons.
What Is The Atomic Number Of…
Using the parts of atom worksheet as a guide, here are some more common atoms for you to build! You can also check the Periodic table for other examples.
- Hydrogen atom has 1 proton, 0 neutrons and 1 electron.
- Helium atoms has 2 protons, 2 neutrons and 2 electrons.
- Carbon atom has 6 protons, 6 neutrons and 6 electrons.
- Nitrogen atom has 7 protons, 7 neutrons and 7 electrons.
- Sodium atom has 11 protons, 12 neutrons and 11 electrons.
- Magnesium atom has 12 protons, 24 neutrons and 12 electrons.
Additional Physics Experiments For Kids
Explore physics, including light, forces, sound and more with one of these hands-on physics experiments below.
Learn about atmospheric pressure with this incredible can crusher experiment.
Explore forces with an easy to set up balloon rocket project.
Pennies and foil are all you need to learn about buoyancy. Oh. and a bowl of water too!
Check out these fun ways to demonstrate capillary action.
Make a pencil float with this easy friction experiment.
Explore sound and vibrations when you try this fun dancing sprinkles experiment.
Make a color wheel spinner to explore light.
Can you light a light bulb with a lemon battery?









